Wednesday, 28 October 2009
Tuesday, 3 February 2009
IV. Viral Pathogenesis
Viral Pathogenesis is the capacity of a virus to cause disease in a target host. It is a sophisticated study about the relationship between viral replication, host defence and viral evasion of the host defence.
I’ll just organise this topic into different parts for easier explaining.
- Sites of Viral Entry into the host.
- Viral spreading in the host body.
- Modes of Transmission.
- Virus-induced injury (cellular).
- Sites of Viral entry into the host.
I shall now start explaining each and every one of these points…
1. Sites of Viral Entry into the host.
There are various places that a virus can enter and start causing disease to the host.
A. Animal host (E.g. Humans, lions, fishes, etc.)
- Skin
- Cuts, abrasions, etc.
- Conjunctiva (eyelids)
- Urogenital tract
- Respiratory tract
- Alimentary tract
B. Plant host
- Any part of the plant as long as there is direct penetration of the cell wall.
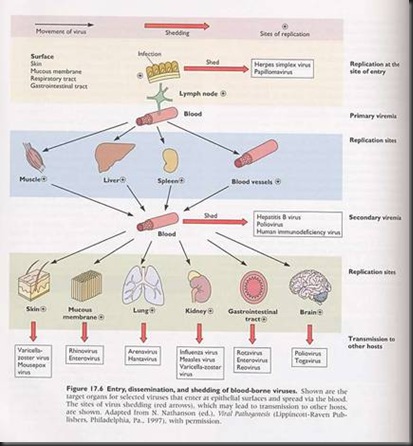
2. Virus spreading in the host body.
i. Systemic infection
- Many organs are infected
ii. Haematogenous spread
- Spread through the bloodstream
- Viremia
- Active / Passive
- Primary / Secondary
iii. Neural spread
3. Modes of Transmission.
- Spread through germ cells
- Consumption of infected tissue
- Respiratory Secretions
- Aerosols during speaking, sneezing, coughing, breathing, singing
- Faeces
- Blood
4. Virus-induced injury (cellular) and effects.
The cells that are infected with viruses will display Cytopathic Effects (CPE).
Some of these effects consist of:
- Altered shape
- Detachment from substrate
- Lysis
- Membrane fusion; syncytium
- Membrane permeability
- Inclusion bodies
- Apoptosis
Other effects shown would be the Formation of Syncytium, shutting off of cell functions and Immunopathological lesions.
a. Formation of Syncytium
Below is a diagram describing Syncytium formation.
b. Shutting off of cell functions
- E.g. poliovirus shuts off cellular function in neurons resulting in cell death and hence paralysis
c. Immunopathological lesions
- Impairment of immune response due to infection of immune cells. (E.g. HIV on CD4+ & CD8+ T lymphocytes)
- Enhancement of immune response causing haemorrhagic fever. (E.g. Dengue haemorrhagic fever, Hantaan, Ebola, etc.)
Viruses? A deadly weapon of war?
Although we may not be sure of being able to prevent such theft, we can learn how to play our part in fighting against it.
We should always make sure to vaccine ourselves and our close family. Also, we should take precautions by keeping our body stystems healthy enough to fight against diseases, by keeping a healthy diet and taking medication whenever needed to.
We can all play a part to fight against viruses, artificial or natural. It is simply a matter of whether we want to. How about you?
Monday, 2 February 2009
Emerging Viruses. How to fight this new foe?
It is good to cure this disease after researching about it, but prevention is better than cure. Therefore, we shall learn about the causes of these emrging viruses, which we must take care to avoid, in order to prevent teh enemy from even having an opportunity to strike, or at least hinder the birth of such problems.
Firstly, we know about virus factors, those that we cannot avoid yet. Spontaneous evolution of a new virus entity and generation of a novel strain due to co-infection of different strains in an individual.
The second part consists of what we can prevent, that is, human factors, such as concentration of people with shared lifestyles, breakdown in public health, climate change, and man invading natural habitats such as cutting of forests to increase their land for development and comfort, which may cause zoonoses.
Men are typically selfish. However, we must understand what must be done, and prevent all of these factors from happening as much as possible, as much as we can survive with.
However, although I believe in doing so, there are cases where certain choices are up to you. Imagine if you are a farmer with sick chickens that are infected with avian flu. With your family starving, would you rather kill the chickens as teh government demands, or sell them illegally for bread and butter. Personally, if we were to die of hunger, I would rather send my children to teh government to take care of, and die of hunger on my own with dignity, since I do know that tehy would want it that way, and that it is better than knowing that all were killed due to my selfishness.
Think about this. What would you do?
Virusoids? What are they?
In this case, I shall be talking about a certain infectious agent known as virusoids that infect plants. Similarly to viroids, they are infectious agents composed exclusively of a single piece of circular single stranded RNA which has some double-stranded areas. It has no capsid or envelope, making it susceptible to certain environment hazards such as heat.
The key is here. Unlike viroids, they do not require assistant virus, as viroids do, such as hepatitis with hepatitis D.
Whether this is better, or if this is not, largely depends on the purpose of the virus. This is what I believe. How about you?
Flaviviridae -II
Flaviviridae
Wow, this was a long post, so I'll be covering on Yellow fever in the next one!! But I realised that something so simple as discarding stagnant water can prevent the breeding of these mosquitos, and eventually, the prevention of such disease. So, get rid of stagnant water, cause Prevention is better that Cure.
Emerging Viruses
Now, how does a virus that infects animals only start infecting humans?? There are various factors, which can be lumped into 2 groups: Virus and Human factors.
Virus Factors:
(i) Spontaneous Evolution
Alright, now, viral populations are hetergenous and there IS a limit of variation, but that is a really big number. Furthermore, as there is selection pressure present, this results in high mutation rates, and thus, spontaneous evolution, where a new virus entity is formed.
(ii)Random Assortment, or the generation of a new strain due to the co-infection of different strains in an individual. That was long, so I'll be using Random Assortment instead.
As previously stated, in the life cycle of a virus, the genetic material of the virus undergoes replication and expression. However, when two viruses infect one cell at the same time, or co-infection, the host cell gets mixed up during the assembly stage and a "new" vius is produced and released out of the cell to infect others. Thus RANDOM assortment
Sunday, 1 February 2009
III. Virus Assembly and Release
In the 3rd stage of the virus life cycle, the new incomplete virus particles start to arrange and assemble themselves in the host cell’s site of viral synthesis – Areas of protein synthesis and processing of virus material in the host cell.
After the assembly process comes to hail when the host cell is exhausted of its nutrients and energy in making the virus proteins and envelopes, the virus particles will begin to leave the host cell via several methods, thus beginning to re-infect other cells again.
A. Virus Assembly
- Depends on the site of Synthesis
- Sites of virus protein synthesis and processing
1. Endoplasmic reticulum
2. Golgi body
- Sites of Assembly
1. Nucleus
2. Endoplasmic reticulum
3. Golgi body
- Viral Envelope sites
1. Nucleus
2. Endoplasmic reticulum
3. Golgi body
4. Plasma membrane
5. Formed via budding off from plasma membrane
A diagram showing a typical budding process.
B. Virus Release
1. Through cell lysis/cell burst/apoptosis.
2. Through budding at the plasma membrane.
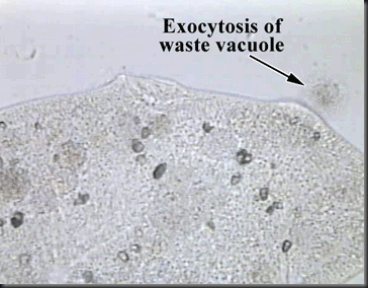
3. Through Accumulation of particles in vesicles and release via Exocytosis.
(Exocytosis is the opposite of Endocytosis,. It is the process of purging unwanted materials from the cell. Below are 2 pictures to show what it is.)
The link to the 2 pictures: http://www.linkpublishing.com/video-transport.htm#Paramecium_-_Exocytosis_
Personal opinion and conclusion...
That would be the gist of what the stage of Virus assembly and release would be talking about.
After going through all the information, I realised that viruses are truly terrifying entities.
Viruses are classified as non-living entity by the central dogma, yet they are able to survive in extremely hard conditions with only some genetic materials, manipulate its host’s mechanism to start reproducing the virus and finally resulted in causing most of the deadly diseases known to man.
Now, is Viruses terrifying or not?
The Original Taxonomy of Viruses
Infectivity is how well the virus infects a host, which quite determines it's strength.
Filterability represents how well it can be filtered through the chamberland filter (refer to previous post on the birth of virology).
Finally, their requirement for hosts, such as which kind, perhaps humans. This determines their flexibility.
Through these, viruses were first classified. Of course, like I mentioned earlier, the road of change is never ending. Who knows, maybe you may be next to change this taxonomy.
Plaques assay
In plaque assay, virus undergoes serial dilution.

http://www.slic2.wsu.edu:82/hurlbert/micro101/images/virus28.gif
I have generally found that plaques assay is not a very good way to find virus concentration. There are many ways for virus to hide, and so this assay is usually an underestimate of the actual number of virus present. For instance, a pfu (plaque forming unit) may not find a cell by the time the attachment period is ended or it may stick to the plate where no cell is present and by the time the monolayer reaches it, it's too far behind to form a visible plaque.
The Birth of Virology
This story begins in 1884, where a scientist, the french microbiologist Charles Chamberland invented teh chamberland filter with pores made smaller than bacteria.
In 1892, the Russian Biologist Dimitri Ivanovski used it to filter the tobacco mosaic virus from infected plant leaves. It was speculated to have been produced by bacteria, but was not further studied. From that point on, it was thought taht such infectious agents could be filtered teh same way and grown on a nutrient medium. This was known as the germ theory.
However, later on, down history, many scientists have done research on this newly discovered type of infectious agents, and helped to change and improve the virology that we study today. But, that's another story.
Indeed, science is ever changing and requires the help of all curious and innovative minds such as those of the students of today, like you and I. That, is why this blog was made. For us to share our knowledge and change our views on microbiology.
Where do Viruses Come From?
Let us start with the regressive theory. It is said that viruses were once parasites, as they were today. Overtime however, genes into required by their parasitism were lost. This is also known as the degeneracy theory. This is very possible, since viruses cannot reproduce without a host.
Next up is the cellular origin theory. This theory states that viruses could have evloved from stray pieces of RNA or DNA, perhaps even plasimds (DNA that moevs between cells, also known as jumping genes). Overtime, they could have developed their capsids and envelopes.
Last but not least, we have the coevolution theory, which states that viruse evolved overtime from pieces of genome and protein.
Personally, I would choose to believe the regressive theory, seeing as how viruses may have been weaken by their activities as parasites. On a side note, this is also an interesting lesson to us. If we depend too much on others, one day, we will be weaken and unable to stand independantly.
This is just my opinion, but how about you? Which theory do you believe in most?
Antibody and Antigen
Antigen - This is the agent that causes your body to produce antibody, as shown by the following picture. It is foreign to the body, i.e. from the external enivoment or and thus, antibodies are formed

Which brings us to.... Antibodies.

For those would like an even more detailed explanation on this subject may click on the following link. I hope that readers have gained an understanding on this. I know the two terms are confusing, I know I've been confused by it a few times before finally getting it.
Enzyme-Linked Immuno-Sorbent Assay (ELISA)
II. Replication and Expression
I shall discuss about the 2nd stage of Virus life cycle, which is the replication of genome, mRNA production, processing and translation. In short, it is known as Viral Replication & Expression.
In actual fact, the way the virus reproduces itself in the host depends on the nature of their respective genome.
For example, Group I of the Baltimore’s Classification - Double stranded DNA viruses (E.G. Smallpox and other viruses from the family Poxviridae), can only replicates in the host cell’s cytoplasm and require host cell polymerases to replicate its genome and therefore is very dependent on the host cell’s cycle and mechanisms. The viral genome contains all factors for genome replication and transcription.
Below is a diagram that describes replication of the genome.
A side note...
Remember Baltimore’s Classification? In studying the subject of viral replication & expression, this classification is important as it is the classification which identifies virus into groups based on their genome & how it is being replicated. This, in turn, gives us a shortcut method of determining how a particular virus replicates as long as we know which class is the virus from.
I realise this after I went back to revise the lecture about viral replication & expression. I also concluded that everything taught to us in the MicroB lectures have significant connection to all the other different aspects in virology. In this case, for example, the Baltimore’s classification can be use to simplify the method which is use to determine how certain type of viruses replicates.
Virus Entry
Even if the virus particle attaches itself onto the cell surface, it does not gain entry into the host cell. There are several routes for the virus to take in order to penetrate into the host cell. Without further ado, I shall explain the routes of entry to the best of my abilities.
I. Attachment and Entry
After the virus attaches itself onto the host cell, there are 2 main routes a virus particle would take to enter an animal host cell and only 1 route in the case of a plant and bacteria host cell. In an animal cell, a virus particle can use....
1. Envelope Membrane Fusion
In envelope membrane fusion, the virus's envelope blends with the cell membrane, releasing its genome into the host cell and starts its replication.
2. Endocytosis
2a. Receptor-Mediated Endocytosis (Envelope)
In receptor-mediated endocytosis, the enveloped virus particle tricks the cell into thinking that it is nothing more than nutrients or harmless proteins. The host cell will then take in the virus particle along with its membrane. Once inside, the virus particle will then release its genome and starts its replication.
2b. Clathrin endocytosis (Naked)
In clathrin endocytosis, the naked virus binds with the host cell membrane to gain entry into the host along with the newly binded membrane from the host.
In a plant and bacteria cell particle, virus particle can only gain entry when there is a breach in their respective cell walls. From there, the virus particle will start to uncoat and starts to infect the host.
Virus Attachment
I. Attachment and Entry
Viruses are non-living entity because they are non-active outside a host cell and do not display actions which define them as living entity by the central dogma but starts to begin motion and actions of living entities once they are inside a host cell.Therefore, infection only starts when the virus is inside of the host cell.
But... how does the Virus get into the cell?
The answer is here!!!
The virus got to attach itself to gain entry to the host cell!!!
Alright... now that you get that... SO, do you know how does the virus attach itself to the cell?
Let me tell you how now!
- Attachment (Virus)
To be able to attach to their respective host cells, every type of viruses has their own specific virus attachment protein (VAP) to the host cell receptor. Therefore, Viruses do not attach itself to all the living cells they come across. They are only able attach themselves to those cells which has specific surface protein receptors that can receive and connect to the viruses’ own attachment proteins.
- Receptors (Host cells)
The receptors can be any surface molecule of the cell. It can be Glycoprotein or Glycolipid. Usually, a cell has multiple receptors and each receptor have their own function and opportunistically, viruses would use it as a mean for attachment. It is also because of that, viruses have a certain host range and tissue tropism.

A typical cell surface (**Note that the glycoprotein and glycolipid are the green structure protruding on the surface)
Now That's all for attachment part. The next post will cover about the routes of penetration by the virus into the host.
Virus-Host Interaction & Virus Life Cycle
As molecular biology students, we know that Viruses are not a living organism defined by the Central Dogma but interestingly, they can still infect living organisms and cause diseases that vary from asymptomatic to death as a result of contracting such viruses. Viruses challenged the boundaries of life science as once doctors and scientists assumed that the only things that cause diseases were only bacteria, fungi and parasites. As Viruses are non-living entity, it would be interesting to look at how they infect the host and therefore cause the disease that they are known to cause.
With this, I’ve decided to share my knowledge as well as giving some of my opinions and comments about lectures on virus life cycle. I hope to get comments for my posts too.
Firstly, I’ve copied the stages of the Virus Life Cycle from the lecture for better understanding of the cycle itself. Below are the stages of the Virus Life Cycle. There are 5 stages all together.
Stages of Virus Life Cycle
I. Attachment and Entry
II. Replication and Expression
o Genome replication
o mRNA production, processing and translation
III. Assembly and Exit
IV. Viral Pathogenesis
Now that the stages of Virus life cycles have been labelled out, I hope that other students out there studying virology like me could get a better idea of the Virus-host interactions and the virus life cycle. This way of classifying the stages helped me in memorising and understanding the whole hoo-haa about Virus-life cycle.
- Attachment
- Penetration
- Uncoating
- Replication & Expression
- Assembly
- Release

Saturday, 31 January 2009
Enveloped DNA Viruses
There are two groups of enveloped DNA Viruses. Below are some interesting information about it.
Herpesviridae
Epstein-Barr virus, frequently referred to as EBV, is a member of the herpesvirus family with Linear ds DNA and three origin of replication.One of the most common human viruses. The virus occurs worldwide, and most people become infected with EBV sometime during their lives. In the United States, as many as 95% of adults between 35 and 40 years of age have been infected. Infants become susceptible to EBV as soon as maternal antibody protection (present at birth) disappears. Many children become infected with EBV, and these infections usually cause no symptoms or are indistinguishable from the other mild, brief illnesses of childhood.
The Epstein-Barr virus appears capable of infecting only two major cell types: the outer (epithelial) cells of the salivary gland, and white blood cells known as B lymphocytes (B-cells). Infection with the Epstein-Barr virus develops first in the salivary gland. Large amounts of the virus are released in the saliva, enabling it to spread from one person to another.
Infection of B-cells with the virus causes them to proliferate. This proliferation is controlled by the immune system; if the correct immune response does not develop, individuals are at risk of developing a form of cancer. It is thought to be responsible for a number of diseases in addition to glandular fever (otherwise known as infectious mononucleosis) and Burkitt’s lymphoma.
A late event in a very few carriers of this virus is the emergence of Burkitt's lymphoma and nasopharyngeal carcinoma, two rare cancers
Most individuals exposed to people with infectious mononucleosis have previously been infected with EBV and are not at risk for infectious mononucleosis. In addition, transmission of EBV requires intimate contact with the saliva (found in the mouth) of an infected person. Transmission of this virus through the air or blood does not normally occur. The incubation period, or the time from infection to appearance of symptoms, ranges from 4 to 6 weeks. Persons with infectious mononucleosis may be able to spread the infection to others for a period of weeks. However, no special precautions or isolation procedures are recommended, since the virus is also found frequently in the saliva of healthy people. In fact, many healthy people can carry and spread the virus intermittently for life. These people are usually the primary reservoir for person-to-person transmission. For this reason, transmission of the virus is almost impossible to prevent.
Symptoms of infectious mononucleosis:
Fever, sore throat, and swollen lymph glands. Sometimes, a swollen spleen or liver involvement may develop. Heart problems or involvement of the central nervous system occurs only rarely, and infectious mononucleosis is almost never fatal. There are no known associations between active EBV infection and problems during pregnancy, such as miscarriages or birth defects. Although the symptoms of infectious mononucleosis usually resolve in 1 or 2 months, EBV remains dormant or latent in a few cells in the throat and blood for the rest of the person's life. Periodically, the virus can reactivate and is commonly found in the saliva of infected persons. This reactivation usually occurs without symptoms of illness. EBV also establishes a lifelong dormant infection in some cells of the body's immune system.
Susceptibility
If antibodies to the viral capsid antigen are not detected, the patient is susceptible to EBV infection.
Primary Infection
Primary EBV infection is indicated if IgM antibody to the viral capsid antigen is present and antibody to EBV nuclear antigen, or EBNA, is absent. A rising or high IgG antibody to the viral capsid antigen and negative antibody to EBNA after at least 4 weeks of illness is also strongly suggestive of primary infection. In addition, 80% of patients with active EBV infection produce antibody to early antigen.
Past Infection
If antibodies to both the viral capsid antigen and EBNA are present, then past infection (from 4 to 6 months to years earlier) is indicated. Since 95% of adults have been infected with EBV, most adults will show antibodies to EBV from infection years earlier. High or elevated antibody levels may be present for years and are not diagnostic of recent infection.
Reactivation
In the presence of antibodies to EBNA, an elevation of antibodies to early antigen suggests reactivation. However, when EBV antibody to the early antigen test is present, this result does not automatically indicate that a patient's current medical condition is caused by EBV. A number of healthy people with no symptoms have antibodies to the EBV early antigen for years after their initial EBV infection. Many times reactivation occurs subclinically.
Chronic EBV Infection
Reliable laboratory evidence for continued active EBV infection is very seldom found in patients who have been ill for more than 4 months. When the illness lasts more than 6 months, it should be investigated to see if other causes of chronic illness or CFS are present.
Hepadnaviridae - Hepatitis
- Partial dsDNA
- Endogeneous DNA - dependent DNA polymerase
- Use of overlapping reading frame
- RNA intermediate
The word "hepatitis" means inflammation of the liver. Toxins, certain drugs, some diseases, heavy alcohol use, bacterial and viral infections can all cause hepatitis. Hepatitis is also the name of a family of viral infections that affect the liver; the most common types in the United States are hepatitis A, hepatitis B, and hepatitis C.
Hepatitis A
Is an acute liver disease caused by the hepatitis A virus (HAV), lasting from a few weeks to several months. It does not lead to chronic infection.
Transmission: Ingestion of fecal matter, even in microscopic amounts, from close person-to-person contact or ingestion of contaminated food or drinks.
Vaccination: Hepatitis A vaccination is recommended for all children starting at age 1 year, travelers to certain countries, and others at risk.
Hepatitis B
Is a liver disease caused by the hepatitis B virus (HBV). It ranges in severity from a mild illness, lasting a few weeks (acute), to a serious long-term (chronic) illness that can lead to liver disease or liver cancer.
Transmission: Contact with infectious blood, semen, and other body fluids from having sex with an infected person, sharing contaminated needles to inject drugs, or from an infected mother to her newborn.
Vaccination: Hepatitis B vaccination is recommended for all infants, older children and adolescents who were not vaccinated previously, and adults at risk for HBV infection.
Acute liver infection
- Immediate symptoms
- Loss of appetiet, nausea, vomiting, fever, abdominal pain and jaundice
- About 5% to 10% of acutely infected adults become chronically infected.
Chronic liver infection
- No visual symptoms
- No abnormalities on laboratory testing
- Some will go on to develop cirrhosis and Hepatocellular carcinoma (primary liver cancer)
Hepatitis C
Is a liver disease caused by the hepatitis C virus (HCV). HCV infection sometimes results in an acute illness, but most often becomes a chronic condition that can lead to cirrhosis of the liver and liver cancer.
Transmission: Contact with the blood of an infected person, primarily through sharing contaminated needles to inject drugs.
Vaccination: There is no vaccine for hepatitis C.
Hepatitis D
Is a serious liver disease caused by the hepatitis D virus (HDV) and relies on HBV to replicate. It is uncommon in the United States.
Transmission: Contact with infectious blood, similar to how HBV is spread.
Vaccination: There is no vaccine for hepatitis D.
Hepatitis E
Is a serious liver disease caused by the hepatitis E virus (HEV) that usually results in an acute infection. It does not lead to a chronic infection. While rare in the United States, hepatitis E is common in many parts of the world.
Transmission: Ingestion of fecal matter, even in microscopic amounts; outbreaks are usually associated with contaminated water supply in countries with poor sanitation.
Vaccination: There is currently no FDA-approved vaccine for hepatitis E.
Tuesday, 27 January 2009
Viral Envelopes. Bad or good?
For one thing, viral envelopes help viruses to enter the host cells, and the glycoproteins on it's surface help to indentify and bind to specific receptor sites on the cell membranes. Thereafter, the viral envelope fuses with the cell membrane, leaving the capside and viral genome free to enter and infect. This is a pretty effective and logical strategy right?
Now, the average joe would probably think that besides it's useful role in binding to cell membranes, it would provides some further protection. But! Did you all know that this envelope makes the virus more susceptible to dessication, heat and detergents, amking the viruses easier to sterilize? Yes, in this case, viral envelopes can be more of a bane than a boon, folks. However, ultimately, whether the viral envelope is needed, depends on the goal of the virus. Does it wish to infect cells that badly? Or does it wish to be more protected? Take your time to think about it.
Thursday, 22 January 2009
Retroviruses
HIV stands for human immunodeficiency virus. HIV destroys certain white blood cells called CD4+ T cells. These cells are critical to the normal function of the human immune system, which defends the body against illness. When HIV weakens the immune system, a person is more susceptible to developing a variety of cancers and becoming infected with viruses, bacteria and parasites.
- envelope with glycoprotein peplomers
- 2 copies of linear plus sense ssRNA, each 7 to 10kb
- 3' polyadenylated tail and 5' cap
- reverse transcriptase (genome doesn't serve as mRNA)

Primary infection
- acute stage
- flu-like symptoms
- fever
- skin rash
- swollen lymph nodes
- rate of replication
- propensity to mutate
- cytopathogenicity
- host resistance (suppresion by CD8 T suppresor cells and presence of cytotoxic T-lymphocytes)
Asymptomatic stage
- no apparent disease
- fall in CD4 T lymphocytes (primary target cell)
- fatigue
- depression
- weight loss
- memory disorders
Largest DNA viruses
The poxviruses are the largest known DNA viruses and are distinguished from other viruses by their ability to replicate entirely in the cytoplasm of infected cells. Poxviruses do not require nuclear factors for replication and, thus, can replicate with little hindrance in enucleated cells. Double-stranded DNA genome and is surrounded by a lipoprotein core membrane. It can remain stable for hours in the air.

Smallpox is caused by the variola virus, a brick-shaped DNA virus in the orthopoxvirus genus. The variola virus is among the largest of all animal viruses and can be seen with a light microscope. The variola virus initially infects the cells in the respiratory tract, then spreads to lymph nodes. The virus enters the bloodstream about 3-4 days after the initial infection.
Eradication of smallpox
The eradication of smallpox occurred with the observation by English physician, Edward Jenner, that milkmaids who developed cowpox, a less serious disease, did not develop the deadly smallpox. In 1796, Jenner took the fluid from a cowpox pustule on a dairymaid's hand and inoculated an 8-year-old boy. Six weeks later, he exposed the boy to smallpox, and the boy did not develop any symptoms. Jenner coined the term "vaccine" from the word "vaca" which means "cow" in Latin. His work was initially criticized, but soon was rapidly accepted and adopted. By 1800 about 100,000 people had been vaccinated worldwide.
Sunday, 18 January 2009
Smallest RNA Viruses
Picornaviridae is the largest virus family (over 230 serotypes)
One linear (+) RNA
5 genera :
- Aphtovirus
- Cardiovirus
- Enterovirus
- heptovirus
- Rhinovirus

Picornaviridae -Rhinovirus
- upper Respiratory tract infection
- serum IgG persists for years
- over 100 rhinoviruses
Symptoms :
Cold
- watery nasal discharge
- congestion
- sneezing
- no fever
Orthomyxoviridae- Influenza Virus
- Enveloped
- Pleomorphic
- Spikes on envelope
- Groups of HA or NA
- Ratio of HA to NA is 5:1
There are three types of influenza virus, A, B and C. A and B types are known to cause flu like symptoms in humans. Type C is rare in humans and only causes mild symptoms.
Genome of Influenza A & B
- ss (-) RNA in 8 segments
- 3 ploymerase polypeptides with each segment
- 5' and 3' end at all segments highly conserved

The Virus strains are characterised by the nature of the two proteins found on there surface, namely Neuraminidase and Hemagglutinin. Approximately 80 percent of the spikes are hemagglutinin, a trimeric protein that functions in the attachment of the virus to a host cell. The remaining 20 percent or so of the glycoprotein spikes consist of neuraminidase, which is thought to be predominantly involved in facilitating the release of newly produced virus particles from the host cell. The inner of the cell contains the RNA nucleotides which are the genetic code for the virus replication.
- 14 subtypes of HA
- 9 subtypes of NA
- HA 1,2,3 and NA 1 and 2 are found in human.
Antigenic Drift and Shift
- Antigenic drift is a mutation in the genetic code of surface antigens (HA/NA) (type A and B)
- Antigenic shift occurs when genes re-assort from different subtypes (only for type A)
Symptoms :
Flu
- fever
- headache
- aches and pains
- fatigue
- exhaustion
- cough
Hierarchy of classification
Hierarchy of classification
Kingdom
Phylum
• Sub-phylum
• Super-class
Class
• Sub-class
• Super-order
Order
• Sub-order
• Super-family
Family
• Sub-family
Genus
• Sub-genus
Species
• Sub-species
There is a specific hierarchy by which all living things are classified
example of the hierarchy - classification of the house cat:
Kingdom: Animalia
Phylum: Chordata
Class: Mammalia
Order: Carnivora
Family: Felidae
Genus: Felis
Species: Felis domesticus
Classification of Humans
Kingdom Animalia :
Usually motile, multi-cellular organisms, without cell walls or
chlorophyll; usually, internal cavity for digestion of nutrients
Phylum Chordata:
Organisms that at one time in their life history have a dorsal
hollow nerve cord, a notochord, and pharyngeal pouches
Class Mammalia:
Warm-blooded vertebrates possessing mammary glands; body
more or less covered with hair; well-developed brain
Order Primates:
Good brain development, opposable thumb and sometimes big
toe; lacking claws, scales, horns, and hoofs
Family Hominidae:
Limb anatomy suitable for upright stance and bipedal locomotion
Genus Homo:
Maximum brain development, especially in regard to particular
portions; hand anatomy suitable to the making of tools
Species Homo sapiens:
Body proportions of modern humans; speech centres of brain
well developed
What is a species: special problems for paleontology
A group of individuals that interbreeds.
A group of individuals that shares a common set of genetic characteristics .
Thursday, 8 January 2009
The Virus Taxonomy
Baltimore Classification System
 The Baltimore classification as previoulsy stated, is based on the viral genome. As shown in the picture, viruses are grouped into 7 groups.
The Baltimore classification as previoulsy stated, is based on the viral genome. As shown in the picture, viruses are grouped into 7 groups. The first group contains viruses that contain positive sense double stranded DNA, (+) dsDNA. which is then translated to mRNA which then codes the protein.
Group 2, where positive sense single strand DNA is transcripted to (+/-) dsDNA before it is then translated to mRNA.
Group 4 is where (+)RNA is transcripted to (-)RNA before it is then transcripted into mRNA.
Group 7 is where (+/-)dsDNA becomes a single strand before it undergoes reverse transcription before it finally becomes mRNA.
That's all for this post.